INTRODUCTION
LESSON PLAN
LECTURE
DIGITAL STORY
ACTIVITY ONE
ACTIVITY TWO
QUIZ
REFERENCES
CONTACT
The
video below is an instructional video on atomic emission spectra,
explaining what they are and how they are created. If you
would like to study a transcript of the video
there is one just below the media player.
Atomic Emission Spectra
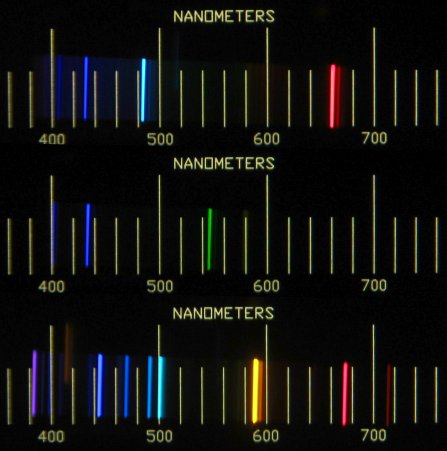
This is an image of three
atomic emission spectra. An atomic emission spectrum is the light
released as a result of atoms absorbing energy. Now that you have
learned a bit about atomic structure, let's investigate how this
relates to the atomic emission spectra.
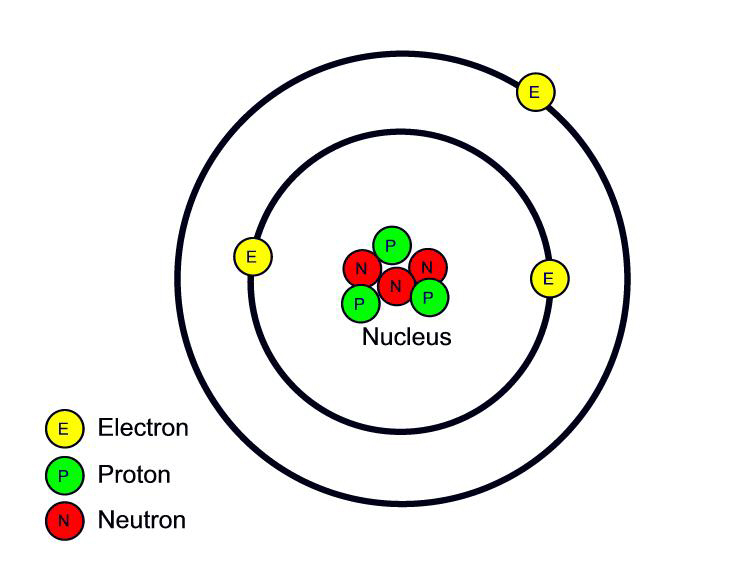
You know that the electrons in
atoms are located in principle energy levels around the nucleus. Right
now we are looking at the Bohr model of the atom since the wave
mechanical model is a little difficult to visualize. Atoms like to be
in the ground state. An atom is in the ground state when all of its
electrons are located in the lowest possible principle energy levels.
An atom can be taken out of the ground state. Let's look at how this
happens.
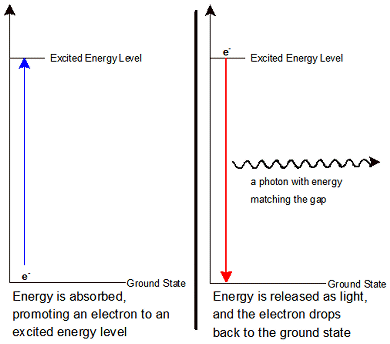
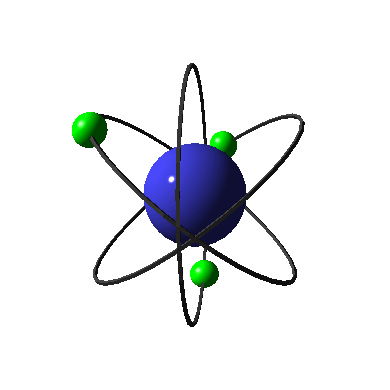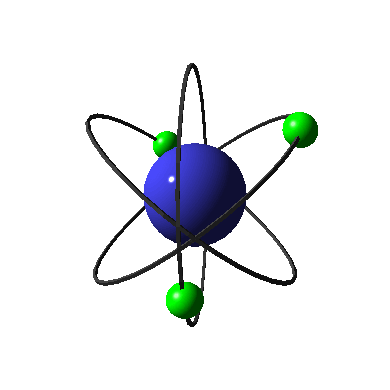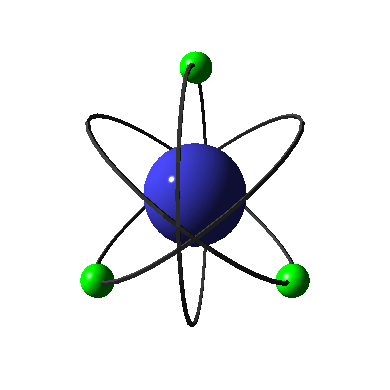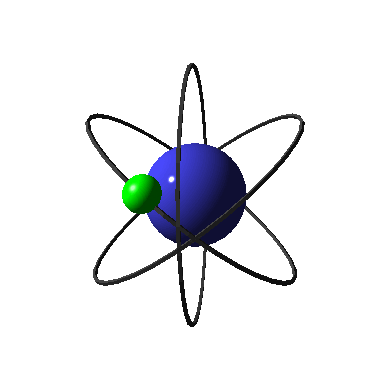
The diagram on the left shows an electron in its ground state. When this electron absorbs energy, it can no longer stay in its current energy level. It has to move into a higher energy level which corresponds to the new amount of energy it holds. When electrons are excited, the atom is unstable. To get back to the stable ground state, electrons must release their energy so that they can move back into the lower principle energy levels. This energy is given off in the form of light, which has an energy that corresponds to the amount of energy given off by the electron. Take a moment to review the diagram of this process, and note that the light or photon given off has an energy that is equal to the difference in the energy between the principle energy level the electron occupies in the excited state, and the one it occupies in the ground state.
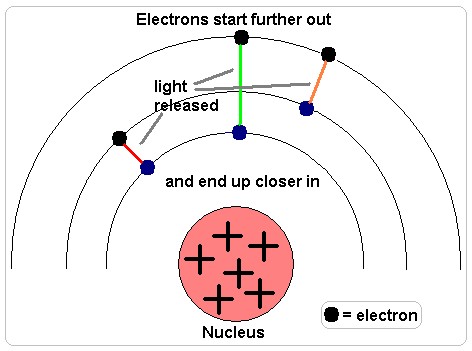
The amount of energy released from the electron controls the color and wavelength of the light emitted. See how in this diagram the electron on the left, which has released the amount of energy needed to transition from the second to the first principle energy level, gives off red light, while the central electron, that has released the amount of energy needed to transition from the third to the first principle energy level, emits green light. Because the energy given off by each electron was different, the color and wavelength of the light given off was different.

Looking back at our first image, we can now recognize that each line on the emission spectra is related to a specific transition made by an electron. The top spectrum is for hydrogen, the middle spectrum is for mercury, and the bottom spectrum if for helium. As you can see, every element has its own unique emission spectrum, which is a consequence of the fact that no two elements have principle energy levels associated with exactly the same energy. In activity two you will have a chance to explore the spectrum for hydrogen and how it is created.
BACK TO TOP
Atomic Emission Spectra



The diagram on the left shows an electron in its ground state. When this electron absorbs energy, it can no longer stay in its current energy level. It has to move into a higher energy level which corresponds to the new amount of energy it holds. When electrons are excited, the atom is unstable. To get back to the stable ground state, electrons must release their energy so that they can move back into the lower principle energy levels. This energy is given off in the form of light, which has an energy that corresponds to the amount of energy given off by the electron. Take a moment to review the diagram of this process, and note that the light or photon given off has an energy that is equal to the difference in the energy between the principle energy level the electron occupies in the excited state, and the one it occupies in the ground state.

The amount of energy released from the electron controls the color and wavelength of the light emitted. See how in this diagram the electron on the left, which has released the amount of energy needed to transition from the second to the first principle energy level, gives off red light, while the central electron, that has released the amount of energy needed to transition from the third to the first principle energy level, emits green light. Because the energy given off by each electron was different, the color and wavelength of the light given off was different.

Looking back at our first image, we can now recognize that each line on the emission spectra is related to a specific transition made by an electron. The top spectrum is for hydrogen, the middle spectrum is for mercury, and the bottom spectrum if for helium. As you can see, every element has its own unique emission spectrum, which is a consequence of the fact that no two elements have principle energy levels associated with exactly the same energy. In activity two you will have a chance to explore the spectrum for hydrogen and how it is created.
BACK TO TOP